
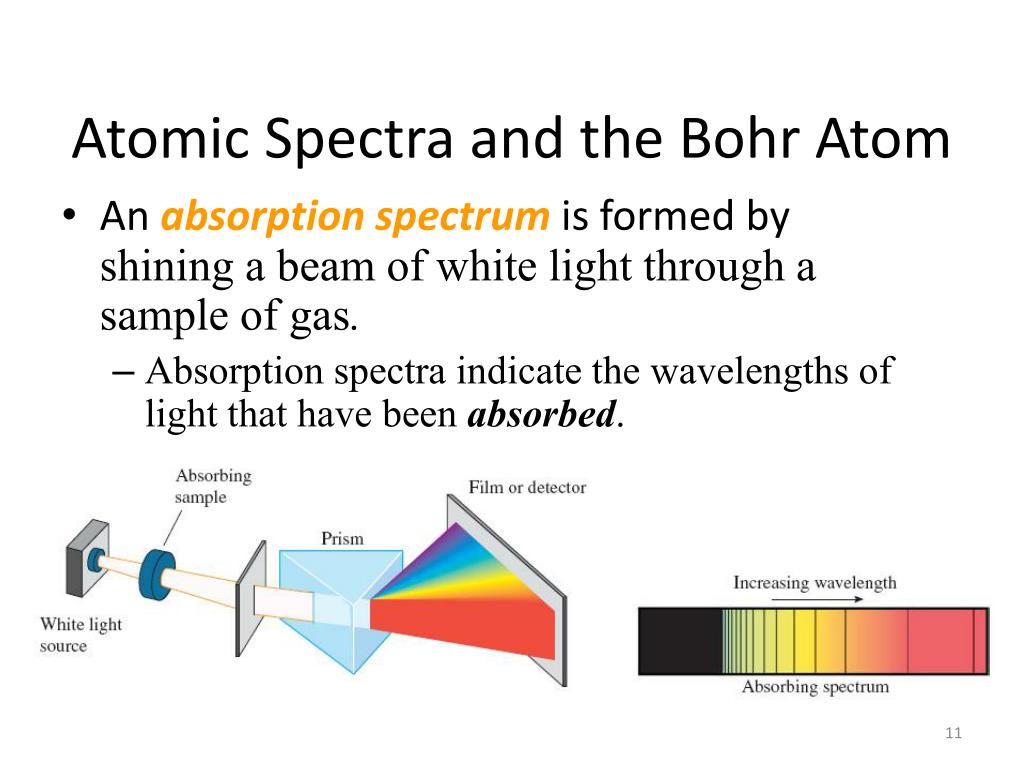
As a result each produces photons with different energy and so the line spectra for different elements will be different. This is not a continuous spectrum as only light of specific frequencies and specific colours are produced.ĭifferent types of atoms have different energy levels.

This causes line emission spectra to be produced, as shown below. The difference between the incident and scattered light frequencies is the vibrational frequency.
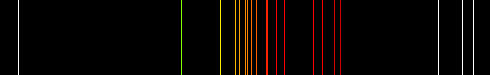
These electrons excite the neon atoms and cause them. This means that each electron transition will produce a photon of a different frequency and hence a different colour. When you apply a high voltage to the electrodes, the neon gas ionizes, and electrons flow through the gas. The spectroscopic data may be selected and displayed according to wavelengths or energy levels by choosing one of the following options: Spectral lines and associated energy levels displayed in wavelength order with all selected spectra intermixed or in multiplet. significant reduction of neon emission spectrum, virtually at one single line. \(f\) is the frequency of light producedĪs the energy levels have different values, each of the possible electron transitions within an atom will produce a photon with a different energy. Welcome to the NIST Atomic Spectra Database, NIST Standard Reference Database 78. namely the energy mediator in metastable atom state and the reaction.The neon light manufacturers use different gases in their. Atomic Spectra Argon spectrum: Argon Hydrogen: Helium: Iodine: Nitrogen: Neon: Mercury: Sodium: At left above is the spectral tube, excited by a 5000 volt transformer. If an electron moves from level \(E_\) the energy of the photon can be worked out using the following: The electrons excite the gas atoms, causing them to emit light, forming a line emission spectrum. The energy of the photon can be worked out using the equation The amount of energy it loses will be equal to the difference in the energy levels it moves between. If an electron is in an excited state it can return to a lower energy level.


 0 kommentar(er)
0 kommentar(er)
